
On June 6, Despite cloudy skies and a tornado watch, over 12,000 intrepid walkers showed up to support the 25th annual AIDS Walk Boston.
The Ragon Institute—and its former iteration, the Partners AIDS Research Center—has supported the AIDS Walk since 1996. The walk begins at the Hatch Shell on the Charles River Esplanade and continues through Boston for a total of 6.2 miles.

Wearing gold crowns in honor of the Ragon Institute’s 14-year sponsorship and fundraising accomplishments, 33 members of the Ragon Institute Team along with family and friends joined this year’s Walk. Several team members also participated in the Larry Kessler 5K run.
All together, the Ragon Institute raised over $11,000 making them the fourth-highest ranking team.
All profits received from the Walk benefit the AIDS Action Committee, a Boston-based non-profit which provides community services to over 2,500 individuals living with HIV/AIDS, conducts statewide prevention programs, and lobbies local, state and federal governments for a fair and effective AIDS policy.
The Ragon Institute continues to demonstrate its commitment, not only to finding a vaccine for HIV, but also to improving the quality of life of persons infected with HIV/AIDS.

When the Ragon Institute was formed in 2009, Director Bruce Walker had a short list of talented AIDS researchers he was determined to engage. On that list was Dan Barouch, Associate Professor of Medicine at Harvard Medical School and Chief of the Division of Vaccine Research at Beth Israel Deaconess Medical Center. Dr. Barouch now serves on the Ragon Scientific Steering Committee as the Director of the Ragon Vaccine and Vector Development program.
Dr. Barouch established himself in 1999 when, along with his mentor, veteran HIV scientist Norman Letvin, he led one of the most prominent AIDS vaccine trials in years. The trial involved immunizing eight monkeys with an experimental vaccine. The unvaccinated monkeys in the control group quickly contracted SIV (simian immunodeficiency virus, a variation of the human HIV virus) and died. Although the vaccinated monkeys also became infected, the vaccine did keep the virus in check for an extended period of time.
However, in one of the vaccinated monkeys, the virus eventually developed mutations which enabled the virus to replicate unchecked by the immune system. This monkey’s entire viral population changed to the mutated strain in less than six weeks and the monkey consequently succumbed to the infection. Armed with this new information reinforcing the importance of the T cell response and the capacity of the virus to mutate, Barouch set out to develop improved novel vaccine strategies.
 The Barouch lab focuses on the immunology of HIV infection to understand what specific responses should be elicited by an ideal HIV vaccine. This understanding is critical to the process of engineering of vaccine components to yield the desired responses.
The Barouch lab focuses on the immunology of HIV infection to understand what specific responses should be elicited by an ideal HIV vaccine. This understanding is critical to the process of engineering of vaccine components to yield the desired responses.
The defining features of any vaccine are antigens, pieces of a virus that the immune system can learn to recognize and combat. A major challenge of HIV vaccination is generating an immune response to the constantly mutating HIV antigens. Therefore, in order to be effective, an HIV vaccine must include antigens that are capable of raising immune responses to a large number of HIV strains.
The Barouch lab is collaborating with the Bette Korber lab of Los Almos National Laboratories to evaluate a new genre of HIV vaccine antigens called “mosaic” antigens. Mosaic antigens are specifically engineered with the problem of HIV mutation in mind. Korber’s group used bioinformatics algorithms to analyze all known sequences of HIV to engineer a “mosaic” sequence that provides immunologic coverage of as many strains as possible. Preliminary studies in non human primates suggest that these novel mosaic HIV antigens out-perform traditional HIV antigens in terms of breadth of immune responses generated.
However, no matter how well designed, antigens on their own tend not to be very effective in stimulating the right balance of immune responses at robust enough levels. Consequently, strategies are needed to augment and shape the immune response to the antigens.
The Barouch lab has explored a series of novel vaccine delivery technologies to do just this. Specifically these technologies fall into two categories: vaccine “vectors” (vehicles that transport antigen into human cells) and “adjuvants” (signaling molecules designed to increase and steer the response to the antigens).
While whole HIV can deliver HIV antigens to cells, it is thought to be too risky for use in a vaccine. Since HIV can mutate so adeptly, it could change from a form engineered to be safe into a form that causes AIDS. Consequently, efforts are underway to instead engineer other, much safer viruses as vectors. Such vectors include vaccinia virus (the virus that is the vaccine for smallpox) and various forms of adenovirus (one of the common virus families that cause cold symptoms).
Historically, the critical problem with the common adenovirus serotype 5 as a vaccine delivery vehicle is that most people have previously been infected with adenovirus and therefore they have high titers of pre-existing antibodies to the virus. These pre-existing antibodies neutralize the viral vector before it has an opportunity to do its job of delivering HIV vaccine antigens.
For this reason, the Barouch lab has instead chosen to develop vectors using strains of adenovirus for which pre-existing antibody titers are either nonexistent or low; preliminary studies in phase 1 studies in humans suggest that these vaccine vectors are safe, well tolerated, and immunogenic.
The lab is also pursuing alternative approaches to deliver the HIV vaccine antigens using DNA or purified protein subunits together with an adjuvant, a signaling molecule designed to increase and steer the response to the antigens.
Dr. Barouch’s lab is also collaborating closely with that of Darrell Irvine, Director of the Ragon Technology Development Program, to evaluate nanoparticles as possible vaccine delivery vehicles in combination with various adjuvants and immunomodulators. Nanoparticle-based immunization strategies may increase the potency of vaccine antigens, or alternatively may allow substantially decreased doses of the vaccine to achieve similar responses.
Developing an effective vaccine against HIV is truly one of the biggest challenges of our time; however most experts now agree that even a vaccine that is only partially effective could reduce the number of infections worldwide and could improve clinical outcomes for those who become infected. Studies performed by Dr. Barouch and colleagues are putting the pieces together to build on our existing knowledge and to bring us closer to an HIV vaccine.
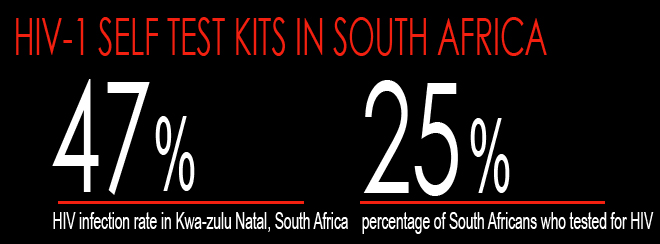
At 18%, South Africa has one of the highest rates of HIV infection of any country in the world.
The province of Kwazulu-Natal has an even higher infection rate of 47%, the highest antenatal infection rate in South Africa. Yet even though testing is free and widely available, only 25% of South Africans pursue testing.
Many defer testing because of the stigma which still persists for those who are HIV positive. An overburdened public health system also results in limited private testing/counseling rooms, long lines, poor service, and lack of confidentiality.
As a result, most patients only start treatment after they have advanced AIDS and symptoms have presented, leading to an unnecessarily high mortality rate. Additionally, a lack of testing prevents treatment which leads to a higher infection rate in the community.

Dr. Krista Dong and Zinhle Thabethe at Edendale Hospital.
Dr. Krista Dong, Massachusetts General Hospital Infectious Disease & HIV specialist and Ms. Zinhle Thabethe have been working in Kwazulu-Natal Province, South Africa, at the front lines of the HIV epidemic for the past decade. They quickly saw a need for a HIV self-test kit which would enable South Africans to perform a test in the privacy of their own home.
Their program the Integration of TB in Education and Care for HIV/ AIDS (iTEACH) based at Edendale Hospital in Pietermaritzburg focuses on innovative “low tech to no tech” approaches to prevention and treatment of HIV and TB infections among persons living in poverty.
The self test kit designed by iTeach is for low literacy populations with easy to follow photos and diagrams. A toll free number is provided which gives the patient the option to call a counselor for assistance with administering the test or to follow up on treatment.

HIV self-test kit prototype
The design of the packaging and instructions are in their final stages, as is the selection of the two types of HIV rapid tests that will be included in the kit.
The kit will soon be tested by a cohort of 100 persons in rural, semi-rural and peri-urban settings. Enrollees will perform a self-test with their results interpreted by a study facilitator in order to confirm interpretation accuracy. It is expected that acceptance of HIV self-testing will be high, as will ability of testers to perform and interpret results.
A successful pilot in this setting will allow for the program to be instituted on a wider scale in similar underserved communities throughout Africa.
‘Testing is the first critical step,” says Dr. Dong, “not just to starting treatment but for prevention too. People have to know their status before they will make any behavioral change so offering a testing method that persons are willing to use is critical.”